Collaboration with VASA Therapeutics : characterization a heart failure model with preserved ejection fraction (HFpEF) / Dahl salt sensitive rats.
Download all informations (pdf)
Financial Disclosure
Artur Plonowski, Benjamin Pratt, Magdalena Krupa, Piotr Lipiński, Christopher Larson, and Mark Herbert are employees and/or shareholders of Vasa Therapeutics.
Role of MMP2, 9 and 13 in Heart Failure
• Elevated plasma MMP2 and 9 in hypertensive diastolic HF patients.
• Upregulated expression and activity in HFpEF comorbidities.
• Increased expression of MMP2, 9, and 13 in heart biopsies from patients with Dilated and Ischemic Cardiomyopathy.
• MMP2 and 9 Tg mice develop fibrotic myocardium.
• MMP2 and 9 KO and prototype pharmacological inhibitors protect from structural changes and functional deficit in animal HF models.
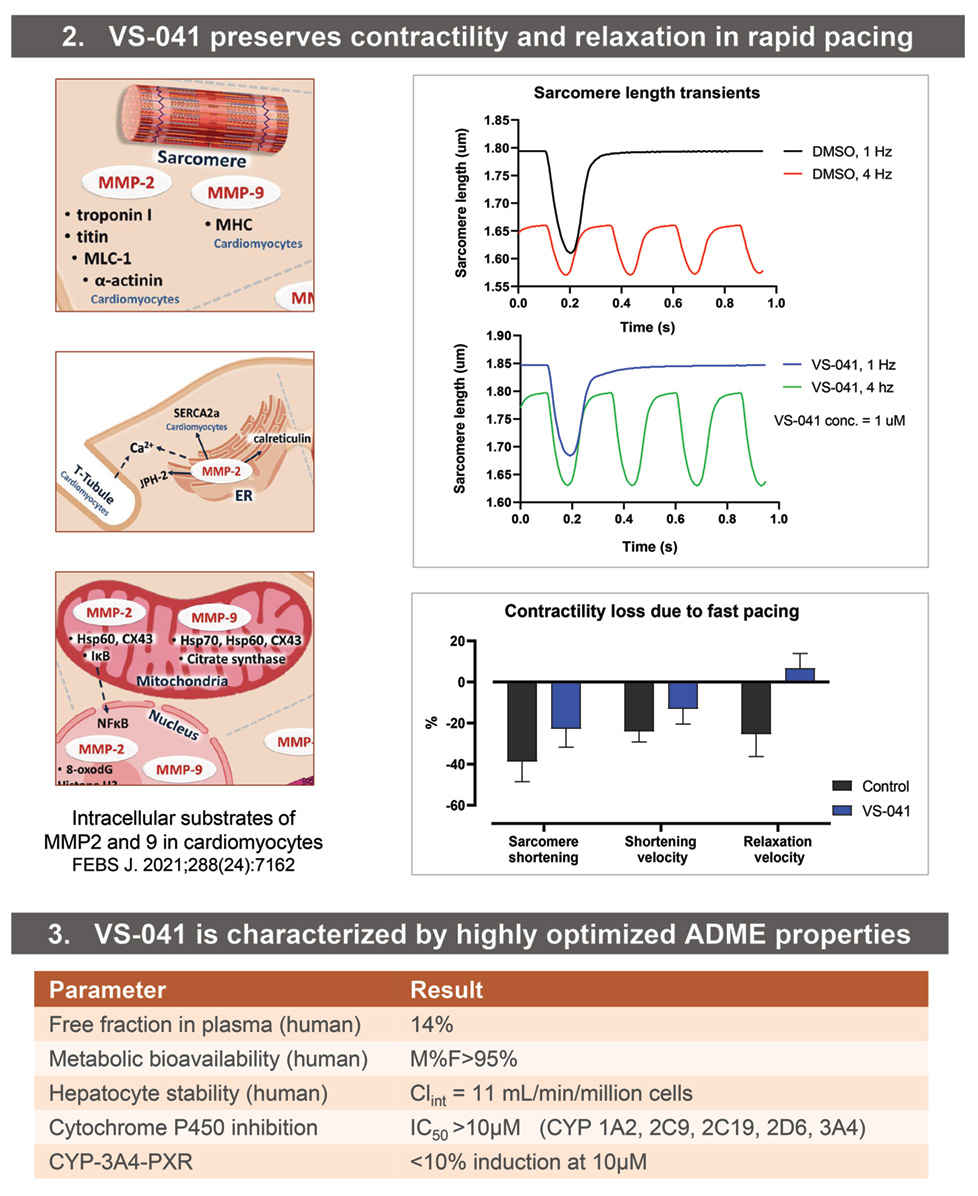
• Intracellular MMP2 and 9 mediate detrimental effects of ischemia, neurohumoral stimulation, hyperglycemia on cardiac contractility, Ca2+ cycling, and bioenergetics.
Plasma MMP2 and MMP9 could serve as biomarkers for precision medicine approach in HFPEF subpopulations.
Aim
Preclinical characterization of VS-041, a novel, orally bioavailable, and selective inhibitor of cardiac-relevant MMP2, 9, and 13, as a potential Clinical Development Candidate for treatment of HFpEF.
Methods
Small-molecule inhibitor of MMP2, 9, and 13 was synthesized by Vasa Therapeutics. Proprietary scaffold was designed and optimized using computer-aided platform (Schrödinger, MA).
In vitro methods
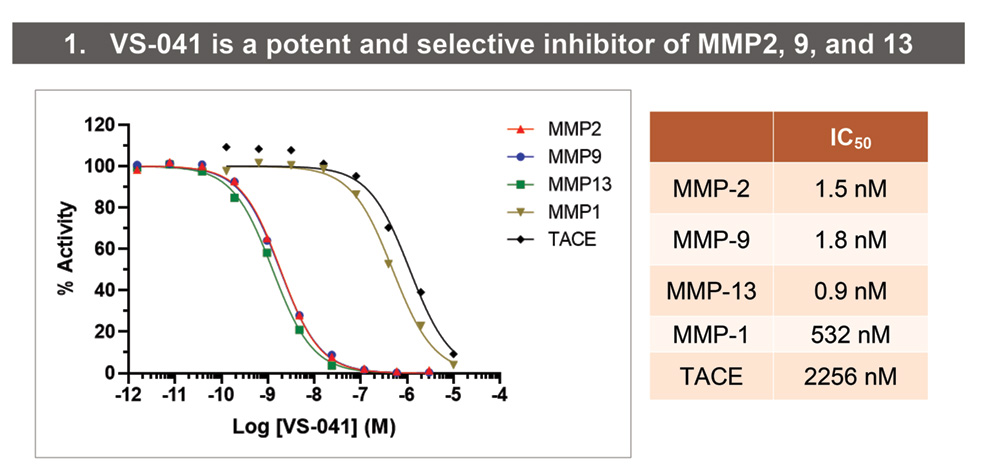
• IC50s against MMP2, 9, 13, MMP1, and TACE were determined using colorimetric assay (MMP2, 9, 13, and 1) or fluorimetric assay (TACE) (Enzo Life Sciences AG, Lausen, Switzerland).
• Sarcomere length transients were measured in adult primary mouse cardiomyocytes using Calcium and Contractility System (IonOptix LLC, Westwood, MA) with digital sarcomere geometry measurement. Cardiomyocytes were paced at 1 and 4 Hz (MyoPacer) in the presence of 1 uM of VS-041 or matched DMSO concentration (0.01%).
• ADME properties were determined using standard assays at Quintara Discovery (Hayward, CA). Protease selectivity was assessed at Reaction Biology Corp., Malvern, PA. In Vitro Safety Pharmacology profiling was determined at Eurofins Cerep (Celle l’Evescault, France).
In vivo methods
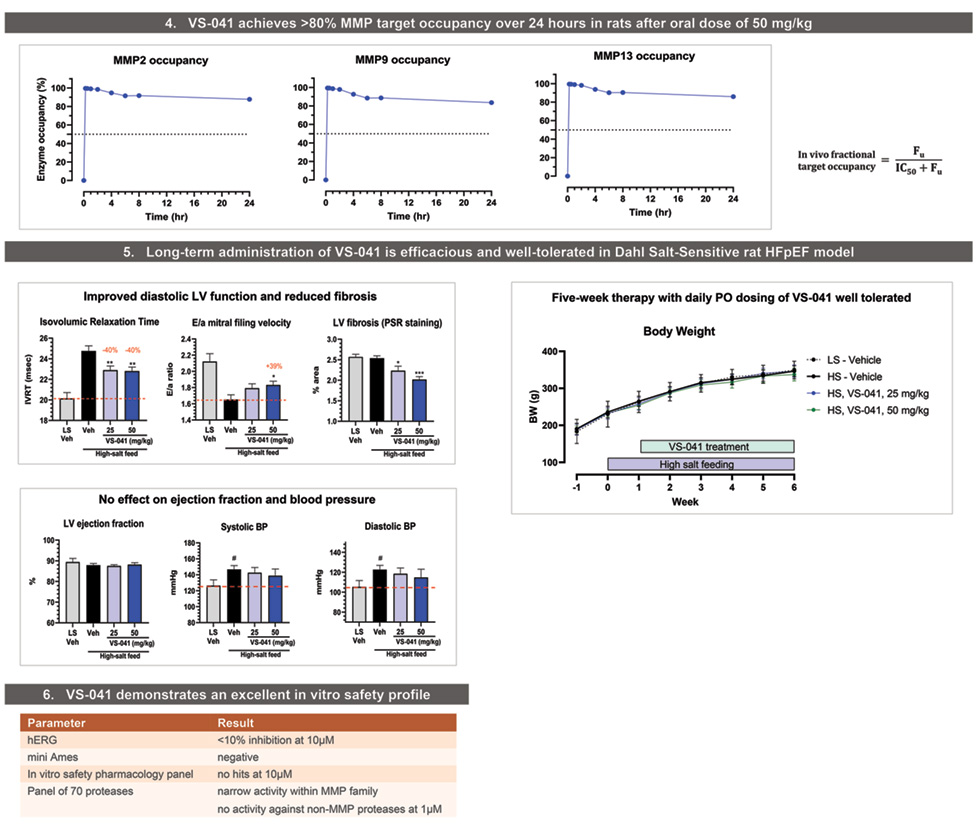
In vivo fractional MMP ooccupancy of VS-041 was determined based on unbound fraction (Fu) plasma exposure in Sprague Dawley rats after oral administration at 50 mg/kg. Compound concentration measured in plasma by fit for purpose LC-MS/MS. Rapid equilibrium of ligand-receptor binding was assumed.
Therapeutic efficacy study in HFpEF and hypertension Dahl-Salt Senisitive rat model
Study was performed by an independent Contract Research Organization (Syncrosome, Marseille, France).
Animals
Three-four-week old (on arrival) Dahl/SS male rats (SS/JrHsdMcwiCrl, Charles River, US), were used for this study. After one week of acclimation, HFpEF groups (n=11-12/group) received an 8% NaCl enriched diet (D05011703, Research Diets). Control group was fed the control diet containing 0.26% NaCl (D10001, Research Diets). Animals included in these experiments were naive to previous administration of any drug.
Echocardiographic analysis
The analysis of 2D-echocardiographic images was performed using the software included in the digital ultrasound system equipped with a 10 MHz phased-array transducer (Vivid 7, GE Medical Systems). All measurements were quantified blindly by a single trained operator and averaged over three consecutive cardiac cycles. Left ventricular (LV) dimensions and wall thicknesses were measured at the level of papillary muscles in M-mode to derive LV ejection fraction (EF). From Doppler spectra of mitral inflow, the isovolumic relaxation time (IVRT) and the peak velocity of early (E) and late (a) filling waves to calculate E/a ratio were measured.
Blood pressure measurement
Rats were deeply anesthetized with isoflurane (induction 4%, maintenance 2-2.5%) associated with buprenorphine (0.03mg/kg, SC) administrated 30 min before induction. Systolic blood pressure (SBP) recordings were performed using a catheter introduced into the carotid artery. Rats were prepared using standard surgical conditions and then intubated and ventilated. The carotid artery was catheterized and SBP recording were performed. A pressure transducer (BLPR (World Precision Instruments, UK) or equivalent) was connected to an amplifier (TBM4M (World Precision Instruments, UK) and a data acquisition unit (Powerlab 16S World Precision Instruments, Australia).
Histological evaluation
Each heart was sectioned at three anatomical transverse planes (Basal (cranial), mid, and caudal or apical ventricular regions) and stained with Sirius red (SR). Sections were digitized using a Nanozoomer (Hamamatsu) and were downloaded and visualized using NDP viewer. Each section was evaluated and individually scored.
Statistical analysis
ANOVA & Tukey Multiple Comp Test: *P<0.05, **P<0.01, ***P <0.001 vs HS. Two-tailed t-test: #P<0.05 vs LS.
The experimental procedures were carried out in accordance with European guidelines for the care and use of laboratory animals (Council Directive 2010/63/UE). The protocol was approved by an Animal Ethical Committee (French National Committee N°71) and by the Higher Education and Research Ministry (APAFIS#32833- 202108191651675 v3).
Results
Summary and Conclusion
- VS-041 is a potent, orally bioavailable and selective inhibitor of cardiac-relevant MMP2, MMP9 and MMP13.
- VS-041 reduces sarcomere contractility deficit due to fast pacing.
- VS-041 demonstrated a robust efficacy in Dahl Salt Sensitive rat model of hypertension and HFpEF (~40% improvement in diastolic function and dose-proportional decrease in LV fibrosis).
- VS-041 had no effect on blood pressure or ejection fraction suggestng a direct effect on diastolic function. No adverse effects were observed after 5 weeks of daily administration.
- Preclinical pharmacology and safety profile of VS-041 support its nomination as a Clinical Candidate and initiation of IND-enabling studies. Phase 1 clinical trial is anticipated in the 2nd half of 2023.
- VS-041 has potential to be first-in-class precision medicine treatment for HFpEF patients with elevated levels of cardiac- relevant MMPs.
Acknowledgements

Project co-funded by European Regional Development Fund and Polish National Centre for Research and Development:
Preclinical development and phase I clinical trial of innovative drug candidate for heart failure with preserved ejection fraction (HFpEF)
(POIR.01.01.01-00-1210/19-01).


